
В кристаллографии , то кубическая (или изометрическая ) сингония является кристаллической системой , где элементарная ячейка находится в форме куба . Это одна из самых распространенных и простых форм кристаллов и минералов .
Есть три основных разновидности этих кристаллов:
- Примитивная кубическая (сокращенно cP и также называемая простой кубической )
- Объемно-центрированный кубический (сокращенно cI или bcc )
- Гранецентрированный кубический (сокращенно cF или fcc , также называемый кубическим плотноупакованным или ccp )
Каждый из них подразделяется на другие варианты, перечисленные ниже. Обратите внимание, что хотя элементарная ячейка в этих кристаллах обычно считается кубом, примитивная элементарная ячейка часто - нет.
Решетки Браве [ править ]
Три решетки Браве в кубической кристаллической системе:
| Решетка Браве | Примитивная кубическая | Телоцентрированный кубический | Гранецентрированная кубическая |
|---|---|---|---|
| Символ Пирсона | cP | cI | cF |
| Ячейка | 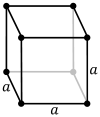 | 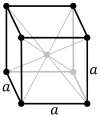 | 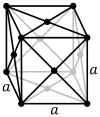 |
Примитивная кубическая система (cP) состоит из одной точки решетки на каждом углу куба. Затем каждый атом в точке решетки поровну делится между восемью соседними кубами, и поэтому элементарная ячейка содержит всего один атом ( 1 ⁄ 8 × 8). [1]
Объемно-центрированная кубическая система (cI) имеет одну точку решетки в центре элементарной ячейки в дополнение к восьми угловым точкам. Он имеет в сумме 2 точки решетки на элементарную ячейку ( 1 ⁄ 8 × 8 + 1). [1]
Гранецентрированная кубическая система (cF) имеет точки решетки на гранях куба, каждая из которых дает ровно половину вклада в дополнение к угловым точкам решетки, что дает в общей сложности 4 точки решетки на элементарную ячейку ( 1 ⁄ 8 × 8 от углов плюс 1 ⁄ 2 × 6 от граней). Каждая сфера в решетке cF имеет координационное число 12. Координационное число - это число ближайших соседей центрального атома в структуре. [1]
Гранецентрированная кубическая система тесно связана с гексагональной плотноупакованной (ГПУ) системой, где две системы различаются только относительным расположением своих гексагональных слоев. [111] плоскость гранецентрированной кубической системы является шестиугольной сеткой.
Попытка создать C-центрированную кубическую кристаллическую систему (т.е. размещение дополнительной точки решетки в центре каждой горизонтальной грани) приведет к простой тетрагональной решетке Браве .
Кристаллические классы [ править ]
В изометрических кристаллической системе имена классов, группы точек (в символах шёнфлиса , Германн-Моген обозначениях , орбиобразию и Кокстер обозначениях ), тип, примеры, Международные Столы для ряда пространственной группы кристаллографии, [2] и пространственные группы , перечислены в таблице ниже . Всего существует 36 кубических пространственных групп.
| # | Группа точек | Тип | Пример | Космические группы | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Имя [3] | Schön. | Intl | Сфера. | Кокс. | Примитивный | По центру лица | По центру тела | |||
| 195–197 | Тетартоидный | Т | 23 | 332 | [3,3] + | энантиоморфный | Ульманнит , хлорат натрия | P23 | F23 | I23 |
| 198–199 | P2 1 3 | I2 1 3 | ||||||||
| 200–204 | Диплоидный | Т ч | 2 / м 3 (м 3 ) | 3 * 2 | [ 3+ , 4] | центросимметричный | Пирит | Pm 3 , Pn 3 | Fm 3 , Fd 3 | Я 3 |
| 205–206 | Па 3 | Ia 3 | ||||||||
| 207–211 | Гироидальный | О | 432 | 432 | [3,4] + | энантиоморфный | Petzite | П432 , П4 2 32 | F432, F4 1 32 | I432 |
| 212–214 | Л4 3 32, Л4 1 32 | I4 1 32 | ||||||||
| 215–217 | Шестигранный | Т д | 4 3 мес. | * 332 | [3,3] | Сфалерит | P 4 3м | Ж 4 3 мес. | Я 4 3 мес. | |
| 218–220 | P43n | F43c | I43d | |||||||
| 221–230 | Hexoctahedral | Oh | 4/m32/m (m3m) | *432 | [3,4] | centrosymmetric | Galena, Halite | Pm3m, Pn3n, Pm3n, Pn3m | Fm3m, Fm3c, Fd3m, Fd3c | Im3m, Ia3d |
Other terms for hexoctahedral are: normal class, holohedral, ditesseral central class, galena type.
Voids in the unit cell[edit]
A simple cubic unit cell has a single cubic void in the center.
A body-centered cubic unit cell has six octahedral voids located at the center of each face of the unit cell, and twelve further ones located at the midpoint of each edge of the same cell, for a total of six net octahedral voids. Additionally, there are 24 tetrahedral voids located in a square spacing around each octahedral void, for a total of twelve net tetrahedral voids. These tetrahedral voids are not local maxima and are not technically voids, but they do occasionally appear in multi-atom unit cells.
A face-centered cubic unit cell has eight tetrahedral voids located midway between each corner and the center of the unit cell, for a total of eight net tetrahedral voids. Additionally, there are twelve octahedral voids located at the midpoints of the edges of the unit cell as well as one octahedral hole in the very center of the cell, for a total of four net octahedral voids.
One important characteristic of a crystalline structure is its atomic packing factor. This is calculated by assuming that all the atoms are identical spheres, with a radius large enough that each sphere abuts on the next. The atomic packing factor is the proportion of space filled by these spheres.
Assuming one atom per lattice point, in a primitive cubic lattice with cube side length a, the sphere radius would be a⁄2 and the atomic packing factor turns out to be about 0.524 (which is quite low). Similarly, in a bcc lattice, the atomic packing factor is 0.680, and in fcc it is 0.740. The fcc value is the highest theoretically possible value for any lattice, although there are other lattices which also achieve the same value, such as hexagonal close packed (hcp) and one version of tetrahedral bcc.
As a rule, since atoms in a solid attract each other, the more tightly packed arrangements of atoms tend to be more common. (Loosely packed arrangements do occur, though, for example if the orbital hybridization demands certain bond angles.) Accordingly, the primitive cubic structure, with especially low atomic packing factor, is rare in nature, but is found in polonium.[4][5] The bcc and fcc, with their higher densities, are both quite common in nature. Examples of bcc include iron, chromium, tungsten, and niobium. Examples of fcc include aluminium, copper, gold and silver.
Multi-element compounds[edit]
Compounds that consist of more than one element (e.g. binary compounds) often have crystal structures based on a cubic crystal system. Some of the more common ones are listed here.
Caesium chloride structure[edit]
The space group of the caesium chloride (CsCl) structure is called Pm3m (in Hermann–Mauguin notation), or "221" (in the International Tables for Crystallography). The Strukturbericht designation is "B2".[6]
One structure is the "interpenetrating primitive cubic" structure, also called the "caesium chloride" structure. Each of the two atom types forms a separate primitive cubic lattice, with an atom of one type at the center of each cube of the other type. In the unit cell of CsCl, each ion is at the center of a cube of ions of the opposite kind, so the co - ordination number is eight. Altogether, the arrangement of atoms is the same as body-centered cubic, but with alternating types of atoms at the different lattice sites. Alternately, one could view this lattice as a simple cubic structure with a secondary atom in its cubic void.
In addition to caesium chloride itself, the structure also appears in certain other alkali halides when prepared at low temperatures or high pressures.[7] Generally, this structure is more likely to be formed from two elements whose ions are of roughly the same size (for example, ionic radius of Cs+ = 167 pm, and Cl− = 181 pm).
The coordination number of each atom in the structure is 8: the central cation is coordinated to 8 anions on the corners of a cube as shown, and similarly, the central anion is coordinated to 8 cations on the corners of a cube.
Other compounds showing caesium chloride like structure are CsBr, CsI, high-temperature RbCl, AlCo, AgZn, BeCu, MgCe, RuAl and SrTl.[citation needed]
Rock-salt structure[edit]
The space group of the rock-salt (NaCl) structure is called Fm3m (in Hermann–Mauguin notation), or "225" (in the International Tables for Crystallography). The Strukturbericht designation is "B1".[8]
In the rock-salt or sodium chloride (halite) structure, each of the two atom types forms a separate face-centered cubic lattice, with the two lattices interpenetrating so as to form a 3D checkerboard pattern. Alternately, one could view this structure as a face-centered cubic structure with secondary atoms in its octahedral holes.
Examples of compounds with this structure include sodium chloride itself, along with almost all other alkali halides, and "many divalent metal oxides, sulfides, selenides, and tellurides".[7] More generally, this structure is more likely to be formed if the cation is somewhat smaller than the anion (a cation/anion radius ratio of 0.414 to 0.732).
The coordination number of each atom in this structure is 6: each cation is coordinated to 6 anions at the vertices of an octahedron, and similarly, each anion is coordinated to 6 cations at the vertices of an octahedron.
The interatomic distance (distance between cation and anion, or half the unit cell length a) in some rock-salt-structure crystals are: 2.3 Å (2.3 × 10−10 m) for NaF,[9] 2.8 Å for NaCl,[10] and 3.2 Å for SnTe.[11]
Other compounds showing rock salt like structure are LiF,[12] LiCl, LiBr, LiI, NaF,[12] NaBr, NaI, KF,[12] KCl, KBr, KI, RbF, RbCl, RbBr, RbI, CsF, MgO, PbS, AgF, AgCl, AgBr[citation needed] and ScN.[13]
The fluorite structure and anti-fluorite structures (AB2) are also Fm3m structures but have a 1:2 ratio of ions. They are designated Wyckoff positions 4a and 8c whereas the rock-salt structure positions are 4a and 4b.[14][15]
Zincblende structure[edit]
The space group of the Zincblende structure is called F43m (in Hermann–Mauguin notation), or 216.[16][17] The Strukturbericht designation is "B3".[18]
The Zincblende structure (also written "zinc blende") is named after the mineral zincblende (sphalerite), one form of zinc sulfide (β-ZnS). As in the rock-salt structure, the two atom types form two interpenetrating face-centered cubic lattices. However, it differs from rock-salt structure in how the two lattices are positioned relative to one another. The zincblende structure has tetrahedral coordination: Each atom's nearest neighbors consist of four atoms of the opposite type, positioned like the four vertices of a regular tetrahedron. Altogether, the arrangement of atoms in zincblende structure is the same as diamond cubic structure, but with alternating types of atoms at the different lattice sites.
Examples of compounds with this structure include zincblende itself, lead(II) nitrate, many compound semiconductors (such as gallium arsenide and cadmium telluride), and a wide array of other binary compounds.
Other compounds showing zinc blende-like structure are α-AgI, β-BN, diamond, CuBr, β-CdS, BP and BAs.[citation needed]
Weaire–Phelan structure[edit]
The Weaire–Phelan structure has Pm3n (223) symmetry.
It has 3 orientations of stacked tetradecahedrons with pyritohedral cells in the gaps. It is found as a crystal structure in chemistry where it is usually known as the "Type I clathrate structure". Gas hydrates formed by methane, propane, and carbon dioxide at low temperatures have a structure in which water molecules lie at the nodes of the Weaire–Phelan structure and are hydrogen bonded together, and the larger gas molecules are trapped in the polyhedral cages.
See also[edit]
- Atomium: building which is a model of a bcc unit cell, with vertical body diagonal.
- Crystal structure
- Dislocations
- Reciprocal lattice
References[edit]
- ^ a b c P. M. de Wolff, N. V. Belov, E. F. Bertaut, M. J. Buerger, J. D. H. Donnay, W. Fischer, Th. Hahn, V. A. Koptsik, A. L. Mackay, H. Wondratschek, A. J. C. Wilson and S. C. Abrahams (1985). "Nomenclature for crystal families, Bravais-lattice types and arithmetic classes. Report of the International Union of Crystallography Ad-Hoc Committee on the Nomenclature of Symmetry". Acta Crystallographica Section A. 41 (3): 278. doi:10.1107/S0108767385000587.CS1 maint: multiple names: authors list (link)
- ^ Prince, E., ed. (2006). International Tables for Crystallography. International Union of Crystallography. doi:10.1107/97809553602060000001. ISBN 978-1-4020-4969-9.
- ^ Crystallography and Minerals Arranged by Crystal Form, Webmineral
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ The original discovery was in J. Chem. Phys. 14, 569 (1946).
- ^ The CsCl (B2) Structure Archived 2008-09-15 at the Wayback Machine
- ^ a b Seitz, Modern Theory of Solids (1940), p.49
- ^ The NaCl (B1) Structure Archived 2008-10-19 at the Wayback Machine
- ^ Sundquist, J. J.; Lin, C. C. (1981). "Electronic structure of the F centre in a sodium fluoride crystal". Journal of Physics C: Solid State Physics. 14 (32): 4797–4805. Bibcode:1981JPhC...14.4797S. doi:10.1088/0022-3719/14/32/016.
- ^ Abrahams, S. C.; Bernstein, J. L. (1965). "Accuracy of an automatic diffractometer. Measurement of the sodium chloride structure factors". Acta Crystallogr. 18 (5): 926–932. doi:10.1107/S0365110X65002244.
- ^ Kao, W.; Peretti, E. (1970). "The ternary subsystem Sn4As3-SnAs-SnTe". Journal of the Less Common Metals. 22: 39–50. doi:10.1016/0022-5088(70)90174-8.
- ^ a b c J. Aigueperse, P. Mollard, D. Devilliers, M. Chemla, R. Faron, R. Romano, J. P. Cuer, "Fluorine Compounds, Inorganic" (section 4) in Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. doi:10.1002/14356007.a11_307.
- ^ Gu, Zheng; Edgar, J H; Pomeroy, J; Kuball, M; Coffey, D W (August 2004). "Crystal Growth and Properties of Scandium Nitride". Journal of Materials Science: Materials in Electronics. 15 (8): 555–559. doi:10.1023/B:JMSE.0000032591.54107.2c. S2CID 98462001.
- ^ "Fluorite". aflow.org. Retrieved 2020-05-22.
- ^ "Rock Salt". aflow.org. Retrieved 2020-05-22.
- ^ L. Kantorovich (2004). Quantum Theory of the Solid State. Springer. p. 32. ISBN 1-4020-2153-4.
- ^ Birkbeck College, University of London
- ^ The Zincblende (B3) Structure Archived October 19, 2008, at the Wayback Machine
Further reading[edit]
- Hurlbut, Cornelius S.; Klein, Cornelis, 1985, Manual of Mineralogy, 20th ed., Wiley, ISBN 0-471-80580-7
External links[edit]
- Basic types of crystal structures
- Body-centered cubic animation
- Face-centered cubic animation

